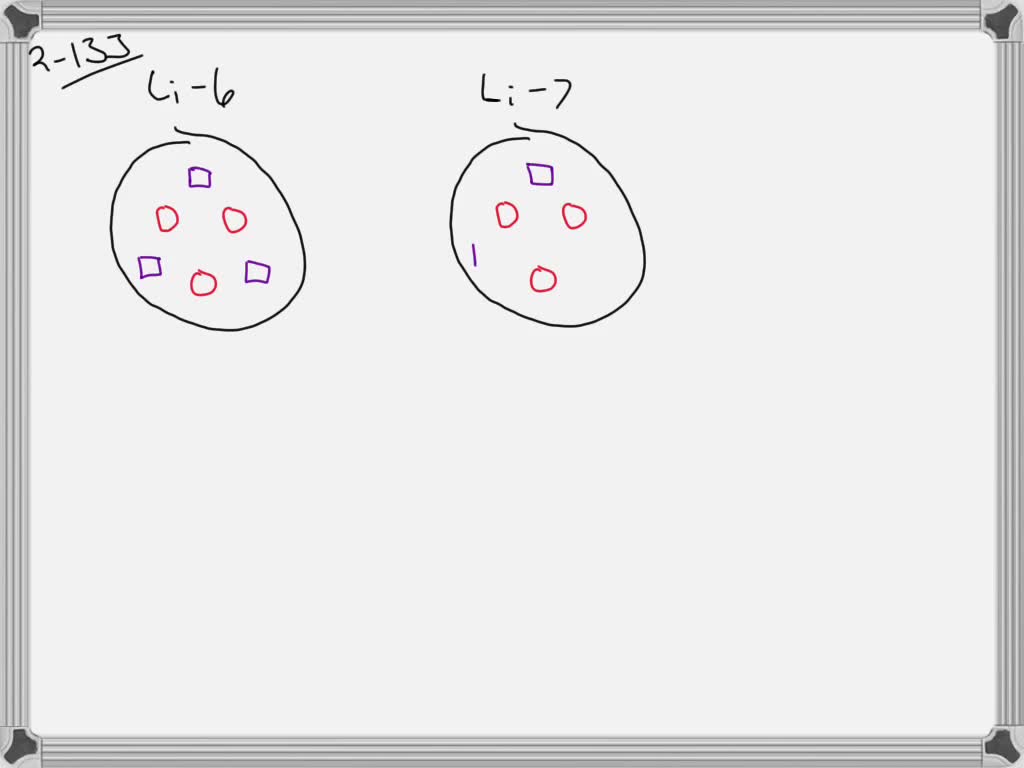
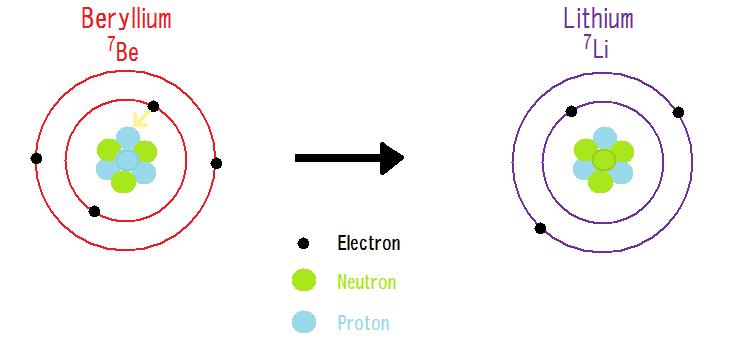
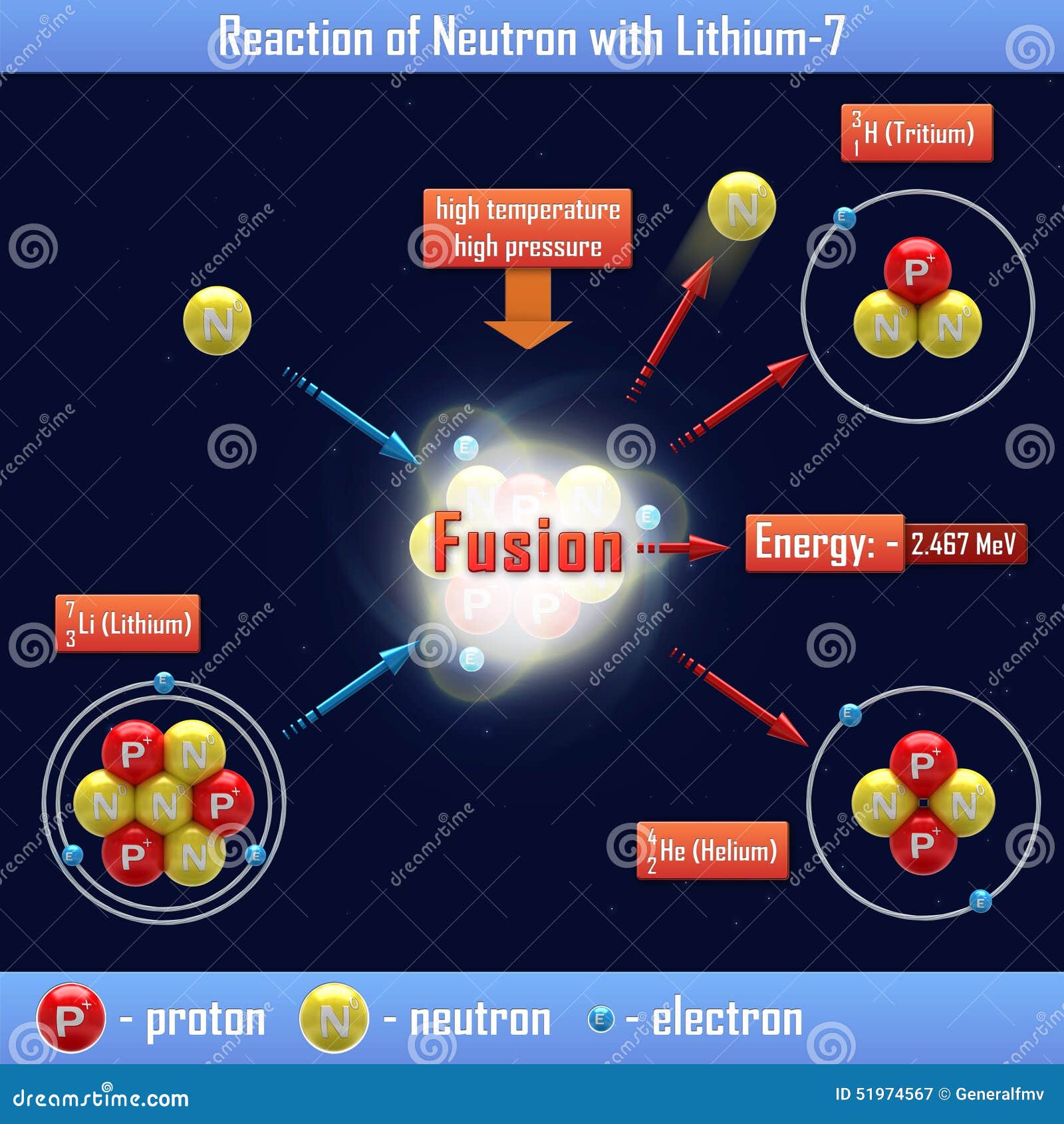
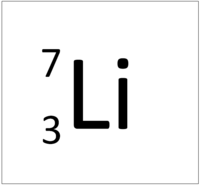
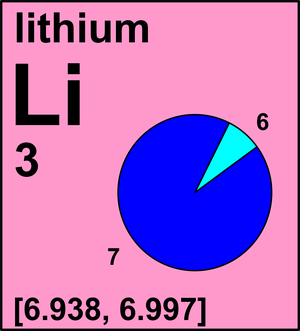
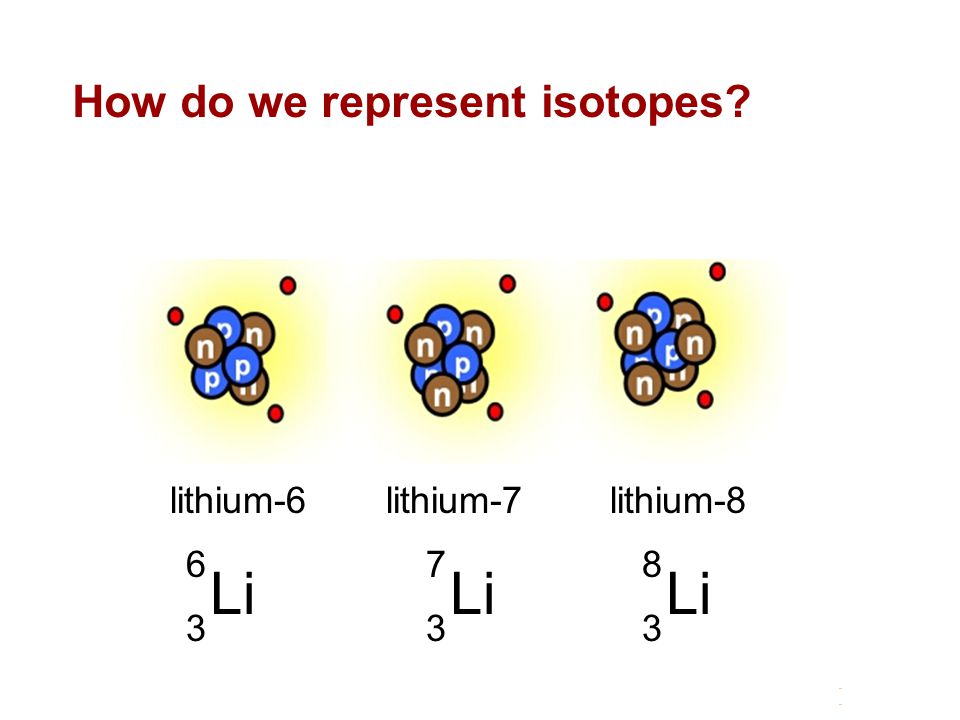
Determine the atomic mass of lithium, given its isotopic composition of 92.4% lithium-7 (mass 7.016 amu) and 7.60% lithium-6 (mass 6.015 amu). | Homework.Study.com

SOLVED: 9. Why are all the values in each row of the table above the same? 10. Lithium has only two stable isotopes. Use the sim to determine the following: a. Atomic

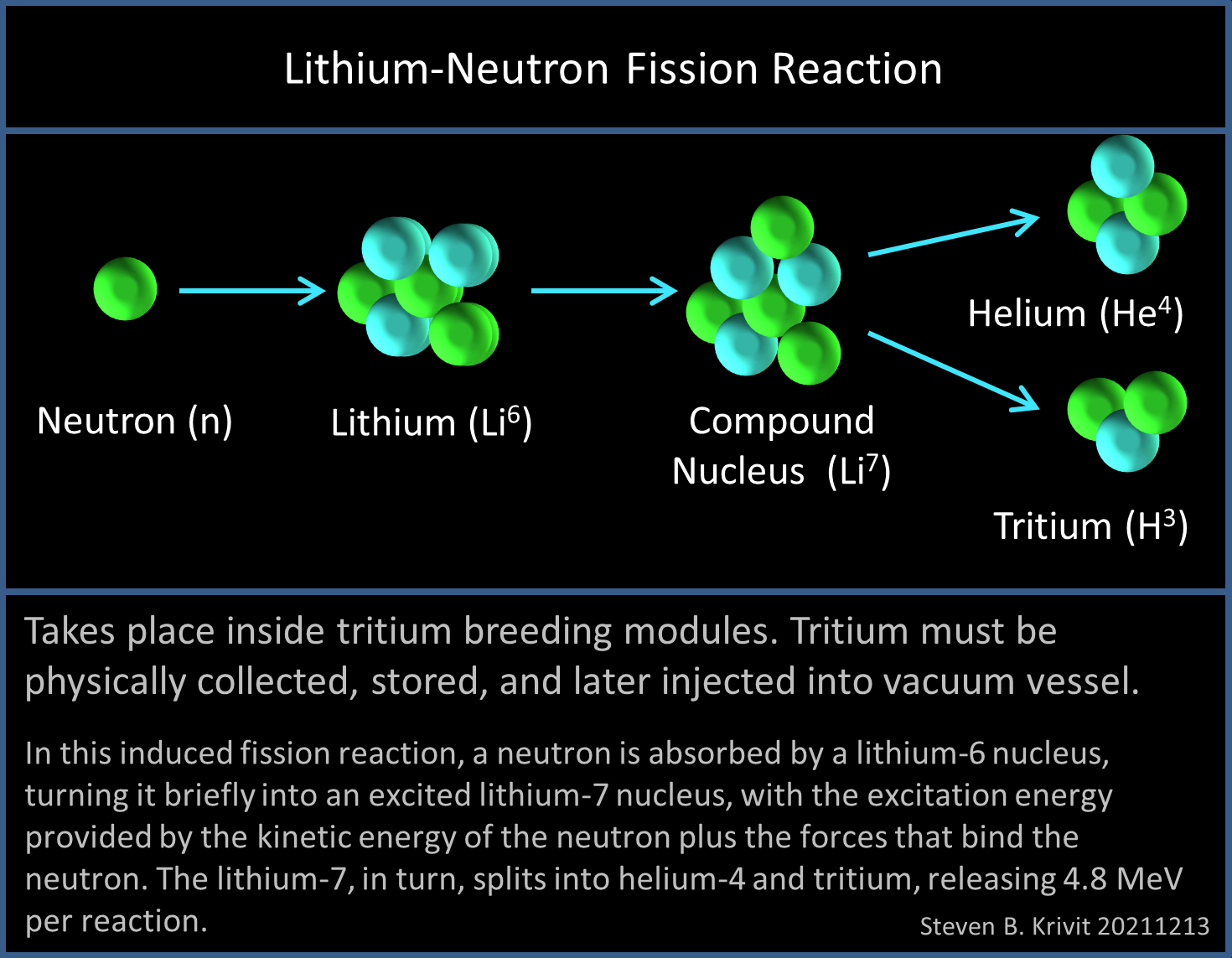
GAO: American Nuclear Plants Vulnerable to Lithium Shortage - News - Nuclear Power News - Nuclear Street - Nuclear Power Plant News, Jobs, and Careers

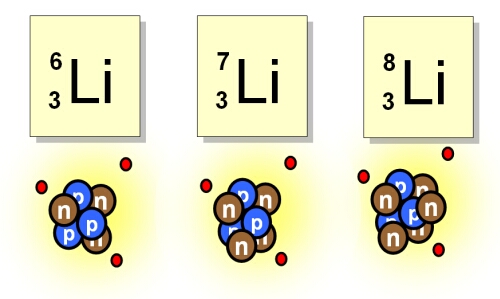
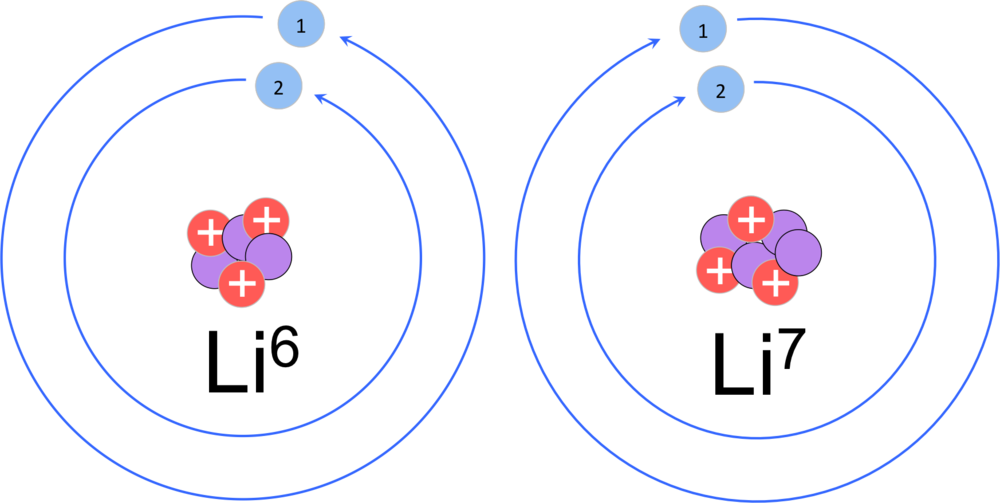
lithiums most common isotopes are lithium 6 and 7 and seven. This also shows its electron configuration | Science projects, Electron configuration, Biochemistry